Corrosion Testing FAQs – 20 most frequent questions and answers
This Corrosion Testing FAQs section assembles the most frequently asked questions we receive from clients and companies regarding different Corrosion Testing procedures and tests.
Here are 20 most frequent Corrosion Testing FAQs
1. What are the most popular corrosion tests?
The most popular corrosion tests methods are ASTM B117 (Standard Practice for Operating Salt Spray (Fog) Apparatus), ASTM G85 (Standard Practice for Modified Salt Spray (Fog) Testing) and ASTM D5894 (Standard Practice for Cyclic Salt Fog/UV Exposure of Painted Metal)
2. What are the differences between ASTM B117, ASTM G85 and ASTM D5894?
ASTM B117 (neutral salt spray test) is arguably the best known and still the most used corrosion test in the world. It was developed back in the early 1940’s and, therefore, has been the reference standard for decades before other methods were developed. Numerous tests methods (like MIL-STD, SAE or even ASTM methods) still refer to ASTM B117 for corrosion testing even if more specific and more advanced test methods are now available. The main reason remains that so many companies around the world have so much data on the behavior of their products and materials based on ASTM B117 that they are reluctant to use newer methods and see decades of collected information become useless or, at least, less relevant.
ASTM G85 can be seen as an improved version of ASTM B117 to some extent. ASTM G85 use the basic principles of ASTM B117 but add the specificities of certain industry sectors in order to address their particularities. The use of a different salt combination (to emulate seawater for example) or a different pH are typical examples.
ASTM D5894 integrates more natural factors than traditional corrosion tests like ASTM B117 or ASTM G85. By integrating the effects of UV, temperature and humidity variations, ASTM D5894 improves the correlation with natural corrosion observed in the field. ASTM D5894 offers a “standard” recipe (ASTM G154 cycle 2 / ASTM G85 annex 5) and more and more industries adapt those test parameters to match their reality. For example, by increasing the UV period you can better simulate Middle East conditions or by changing the electrolyte composition, urban environment may be more realistically mimicked.
3. What is the difference between a Corrosion Test and a Cyclic Corrosion Test?
A cyclic corrosion test, as its name indicates, cycles between:
- 2 or more test conditions (ASTM G85 Annex 5 alternates between Fog and Dry-off periods)
- 2 or more environments (ASTM D5894 alternates between UV exposure, Cyclic salt Fog and sometimes a cold period)
On the other hand, a “classic” corrosion test is a steady-state condition. The best example is ASTM B117 where the specimens are continuously exposed to a fog of 5% NaCl water solution.
4. How long does a corrosion test last?
Just like their colleagues from the weathering community, corrosion experts have tried to find with no great success that magic number. No matter how the question is formulated, the answer is always the same: “It depends!” It depends for one simple reason: Outdoor conditions cannot be controlled the way lab conditions are! While doing corrosion testing, we use calibrated salt fog chambers, pure and specific salts combination, very accurately controlled atmosphere (humidity and temperature), monitored pressure and spray flows, purified water, and predefined cycles of spray and dry-off periods. On the other hand, outdoor exposure depends on when and where you are: waterfront, humidity, smog, rain, temperature, pollution, chemicals, etc., All those parameters have a major influence on the “efficiency” of outdoor corrosion.
Corrosion tests were designed as comparative tools. When you expose 2 materials in controlled lab conditions (that are a good simulation of their expected life conditions), the product that shows the best performance in the lab will also have the best performance in the field.
Please find below some common test durations used in various industry sectors:
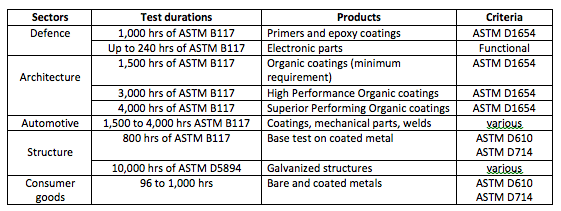
Nobody can (or should) tell you that 300 hrs of ASTM B117 represents 2 months, 1 year or 3 years for your very own product and its unique environment. There are too many factors involved to give such a precise prediction.
5. What is the acceleration factor of a corrosion test?
See “How long does a corrosion test last?”
6. What are the most popular evaluations performed during a corrosion test (internal testing)?
The most popular evaluations performed on coated samples are:
- ASTM D610 (Degree of rusting)
- ASTM D714 (Degree of blistering)
- ASTM D1654 (Evaluation of coated specimens)
- ASTM D3359 (Tape adhesion test)
- ASTM D3363 (Film hardness)
- ASTM D4541 (Pull-Off strength)
7. What is internal testing and why do it?
8. I want to simulate 2, 5 or 10 years of natural exposure, how long will it take [in accelerated lab conditions]?
See “How long does a corrosion test last?”
9. What is the difference between the terms Sample and Specimen?
In the Testing Industry, a sample is a type of product and a specimen is an individual unit. In other words, specimens are identical “items” and samples are different “items”.
For example, if I have 4 identical apples in front of me, I have 4 specimens of the same sample. On the other hand, if I have 1 apple, 1 orange and 1 peach: I have 1 specimen of 3 (different) samples.
Samples and specimens are arguably the most accurate terms to use to avoid any confusion but synonyms are also used:
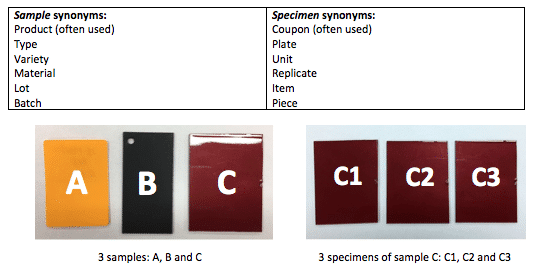 10. How many specimens of each sample should I test?
10. How many specimens of each sample should I test?
Non Destructive Testing
If only non destructive evaluations are performed (Degree of rusting or blistering for example), you can submit as little as 1 single specimen per sample. In that case the same specimen will go all the way to the end of the study.
If you want to remove specimens at specific intervals, the minimum number of specimens will depend on the total duration of your study and the number of intervals you have.
Destructive Testing
If destructive (or damaging) evaluations are requested at several intervals (Scribing, bending, adhesion), the number of specimens needed will have to be calculated.
11. What are the standard dimensions of a test specimen?
The dimensions for a standard test specimen are as follows: 3’’ wide x 6’’long, flat and no thicker than 1/8’’. Note that some test methods (like MIL-DTL-53022E) require specific specimen dimensions. However, depending on your needs, we can accommodate 3-dimensional samples and much larger samples. If you cannot provide standard samples, our experts will work with you on the most cost-effective approach.
12. Can I test larger (or 3D) samples?
Micom has the capability to accommodate large 3-dimensional samples. Contact us to discuss your specific needs.
13. What is corrosion?
Corrosion is the degradation of a material that involves electrochemical reactions. The purpose of that process is to form more stable compounds such as oxides or hydroxides. Stable compounds are formed by mutually attracted ions. This electrochemical process occurs on metals, polymers or ceramics but the term corrosion is only used when related to metals. For other categories of materials, the term degradation is preferred or at least, more commonly used.
Rust is definitely the most known corrosion product. So much that the terms rusting and corrosion are sometimes falsely interchanged but rust only relates to iron. The scientific name of rust is iron oxide. When exposed to oxygen (including moisture or water), iron and iron alloys react with oxygen to form iron oxides. Rust (iron (III) oxide) is one of them and is the dominant form of natural iron oxide. A metal without iron do not rust…it corrodes!
Corrosion occurs on exposed surfaces, it is a diffusion process. That is why it is crucial to coat surface to prevent corrosion. Paint, passivation, galvanization or electrodeposition are different types of coating techniques that will present/slow down the corrosion process.
14. What is prohesion?
The prohesion test is actually the protocol described in ASTM G85 annex 5 (Dilute Electrolyte Cyclic Fog/Dry Test). The term Prohesion is still widely used to describe that test and comes from “PROtection is adHESION).
15. What are the different types of corrosion?
The most common types of corrosion are:
- Uniform corrosion
- Stress Corrosion Cracking (SCC)
- Galvanic corrosion
- Crevice corrosion
- Pitting corrosion
16. What is galvanic corrosion?
Galvanic corrosion is the type of corrosion that occurs when 2 different metals are put in contact via an electrolyte. The stronger the electrolyte, the more efficient is the electronic exchange between the 2 metals. Pure water is a weak electrolyte since only a small fraction of the H20 molecules will ionize. On the other hand, salted water is a stronger electrolyte since all the NaCl will break up in Na+ and Cl– ions until it reaches saturation.
Each metal has a certain electrochemical potential in a specific electrolyte, i.e. a natural tendency to oxidize. The potential difference between the 2 metals will dictate which metal will be “attacked”. The metal with the lowest electrochemical potential will be oxidized (corroded). 2 common examples are shown below:
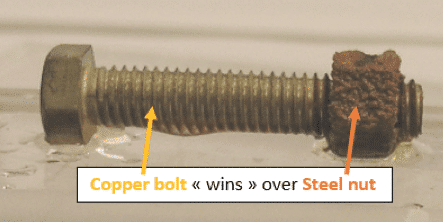
Example #1: Copper “wins” over steel, therefore oxidation occurs on the steel nut (anode)
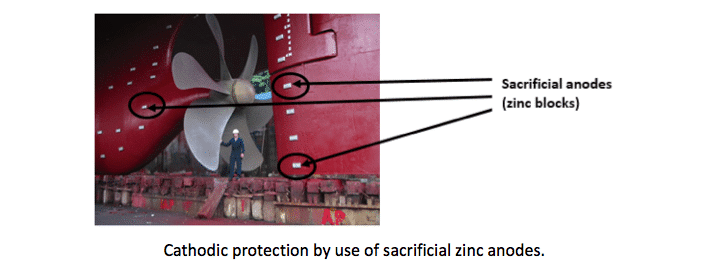
Example #2: Steel “wins” over zinc, therefore zinc (anode) will be oxidized. A common use of this natural reaction is galvanization where zinc is intentional put on steel to prevent rusting. This process is also known as sacrificial anode or cathodic protection.
 17. What is uniform corrosion?
17. What is uniform corrosion?

When corrosion occurs over the entire surface, it is called uniform (or general) corrosion. A steel plate placed in a salt spray chamber will exhibit uniform corrosion. The rate of weight loss can be used to quantify the rate of degradation.
The committee in charge of developing ASTM B117 actually used uniform corrosion to determine the precision of the test method. Repeatability and reproducibility were determined by exposing sets of steel plates in several labs and then compare the various rates of weight loss.
18. What is crevice corrosion?

Crevice corrosion is a localized corrosion at the intersection of a joint or along 2 adjacent surfaces. Contrary to galvanic corrosion, it does not require both surfaces to be metals. Crevice corrosion can be observed at the junction of a metal and a polymer if, for example, accumulation of water occurs.
19. What is pitting corrosion?

When corrosion creates holes/pits at the surface of a metal, it is called pitting corrosion. Pitting can be induced by mechanical damage at the surface, a poorly applied coating or aggressive chemical like chlorides. Pitting is a very destructive form of corrosion and is usually difficult to notice as it is often covered by oxides.
20. What is stress cracking?
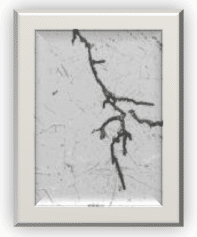
Stress cracking is not limited to metals. It can occur on polymers (solvents attack) and elastomers (ozone cracking). In metallurgy, alloys are more prone to SCC than pure metals. SCC is the propagation of cracks in a corrosive environment. When affected by SCC, ductile metals can behave like brittle metals since the propagation of cracks can lead to sudden breakage. As its name indicates, by reducing the stress in a structural component, this type of corrosion can be minimized.
We hope these Corrosion Testing FAQs will help you better understand the overall Corrosion Testing. If you have any questions about Corrosion tests, we invite you to contact us today. It will be our pleasure to answer your questions and help you with your Corrosion testing custom requirements.


